Research & Development
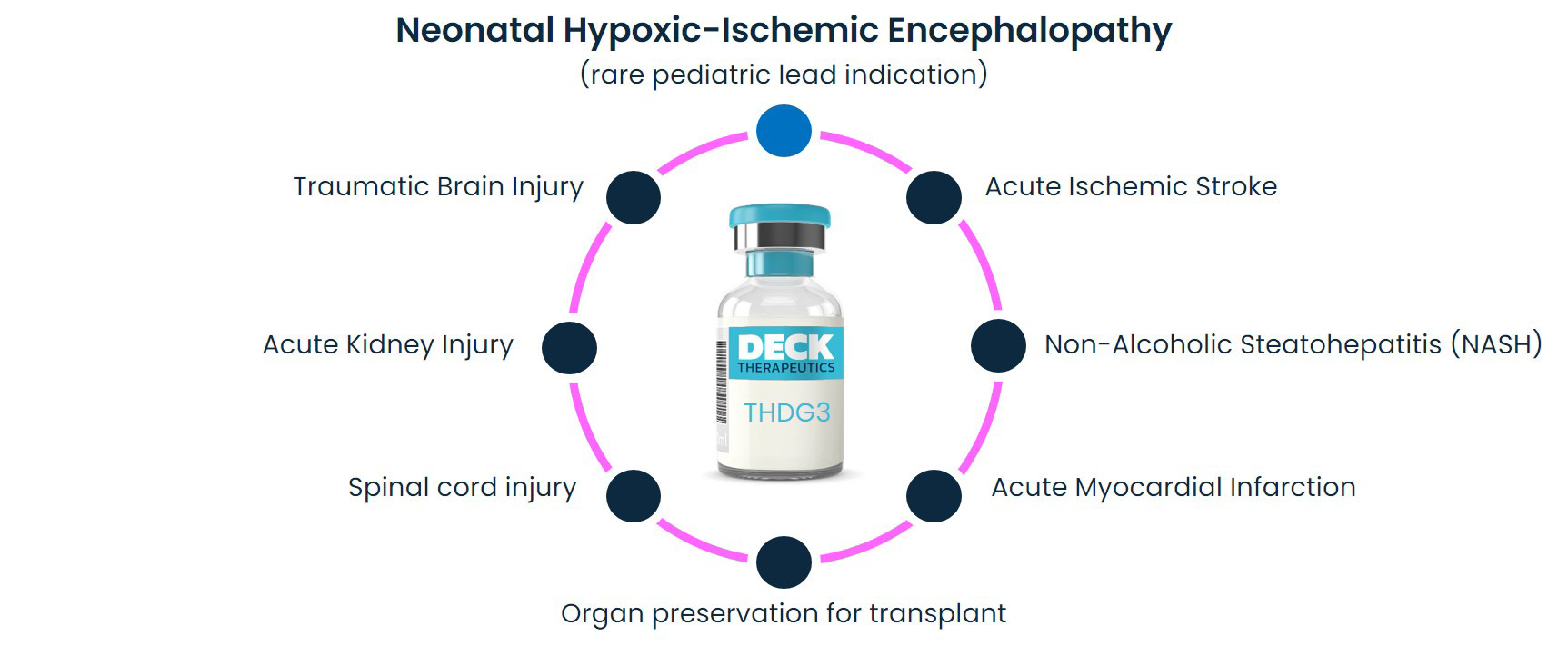
DeckTherapeutics aims to develop THDG3 in multiple indications in which the brain or other tissues are injured following hypoxia-ischemia or ischemia-reperfusion.
DeckTherapeutics
Pipeline
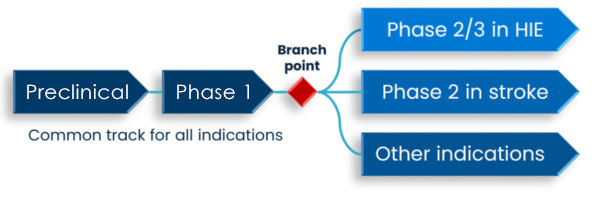
THDG3 is currently being progressed through preclinical and subsequently phase 1 in a common track intended to support dosing in patients in a panel of indications.
DeckTherapeutics will develop THDG3 in the rare pediatric disease HIE internally and potentially partner THDG3 in other indications.
Regulatory incentives in HIE
DeckTherapeutics will file for Orphan Drug Designation (ODD) and for Rare Pediatric Disease (RPD) designation for THDG3 in the rare pediatric condition HIE, potentially resulting in grant of a Rare Pediatric Disease Priority Review Voucher by the US FDA upon marketing authorization.
Furthermore, DeckTherapeutics anticipates that THDG3 will qualify for other regulatory incentives such as FDA Fast Track and Breakthrough designations and EMA Prime designation. We will work closely with the relevant regulatory bodies through-out the development phase.
ODD is usually granted to therapeutics developed for life-threatening or chronically debilitating diseases for which no satisfactory treatment exists and which affect few fewer than 200,000 people per year in the U.S. or fewer that 5 in 10,000 in the EU.
Orphan drugs usually enjoy 7 years (US) or 10 years (EU) of market exclusivity, prescription drug user fee waivers and tax credits for qualified clinical trials.
Partnering
DeckTherapeutics’ omega-3 platform has a wide range of potential applications in reducing tissue damage following a critical event. We welcome inquiries about potential collaborations or partnerships that would aim to leverage our expertise and IP to create new treatment options and value.

